Who am I?
I am a Scientist with a PhD in Molecular Medicine, specialized in lipid and intestinal metabolism, and non-coding RNAs. I have collaborated with international teams at the University of Milano and the University of Uppsala, enhancing my scientific and cross-cultural communication skills. Passionate about science communication and writing, I run the Instagram page @biology_pills_, where I simplify complex biological concepts in Italian to engage a broader audience. My goal is to bridge the gap between research and the public, making science more accessible and inspiring curiosity.
My Latest Work
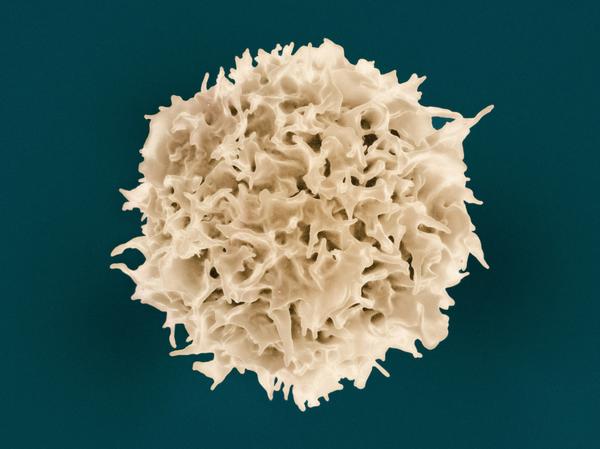
Lipid-associated macrophages between aggravation and alleviation of metabolic diseases
Lipid-associated macrophages (LAMs) are phagocytic cells with lipid-handling capacity identified in various metabolic derangements. During disease development, they locate to atherosclerotic plaques, adipose tissue (AT) of individuals with obesity, liver lesions in steatosis and steatohepatitis, and the intestinal lamina propria. LAMs can also emerge in the metabolically demanding microenvironment of certain tumors. In this review, we discuss major questions regarding LAM recruitment, differentiation, and self-renewal, and, ultimately, their acute and chronic functional impact on the development of metabolic diseases. Further studies need to clarify whether and under which circumstances LAMs drive disease progression or resolution and how their phenotype can be modulated to ameliorate metabolic disorders.
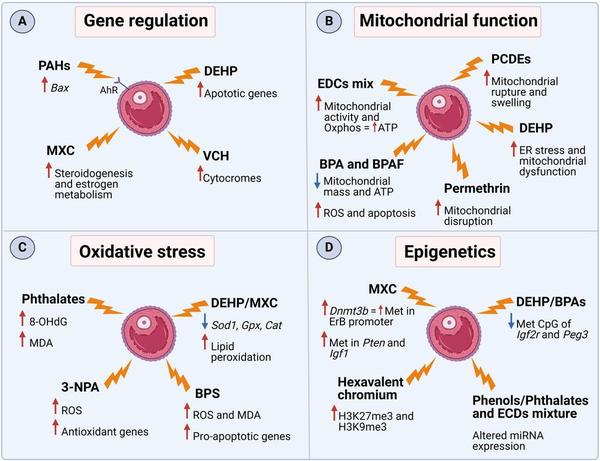
Which is the current knowledge on man-made endocrine- disrupting chemicals in follicular fluid? An overview of effects on ovarian function and reproductive health
The increase in female reproductive disorders, such as polycystic ovary syndrome, endometriosis, and diminished ovarian reserve that lead to subfertility and infertility, has encouraged researchers to search and discover their underlying causes and risk factors. One of the crucial factors that may influence the increasing number of reproductive issues is environmental pollution, particularly exposure to man-made endocrine-disrupting chemicals (EDCs). EDCs can interfere with the ovarian microenvi...
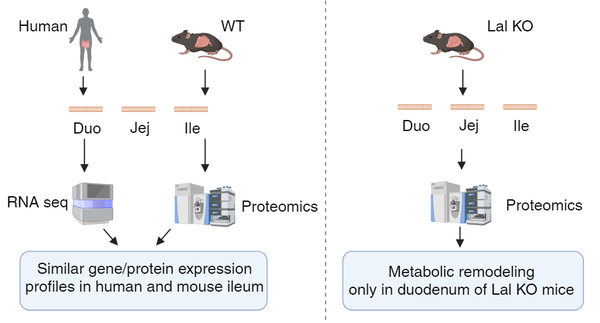
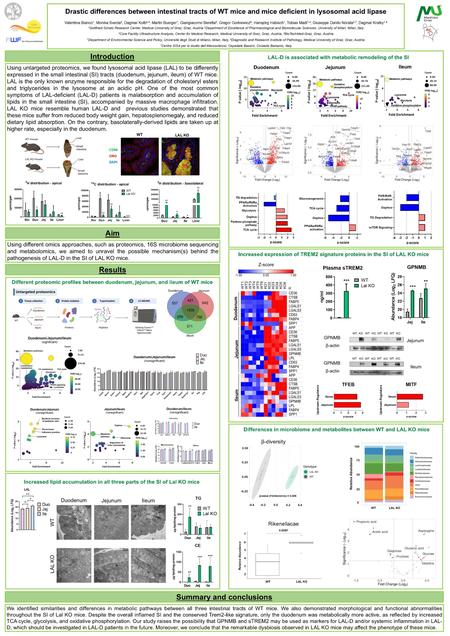
Regional Differences in the Small Intestinal Proteome of Control Mice and of Mice Lacking Lysosomal Acid Lipase
The metabolic contribution of the small intestine (SI) is still unclear despite recent studies investigating the involvement of single cells in regional differences. Using untargeted proteomics, we identified regional characteristics of the three intestinal tracts of C57BL/6J mice and found that proteins abundant in the mouse ileum correlated with the high ileal expression of the corresponding genes in humans. In the SI of C57BL/6J mice, we also detected an increasing abundance of lysosomal acid lipase (LAL), which is responsible for degrading triacylglycerols and cholesteryl esters within the lysosome. LAL deficiency in patients and mice leads to lipid accumulation, gastrointestinal disturbances, and malabsorption. We previously demonstrated that macrophages massively infiltrated the SI of Lal-deficient (KO) mice, especially in the duodenum. Using untargeted proteomics (ProteomeXchange repository, data identifier PXD048378), we revealed a general inflammatory response and a common lipid-associated macrophage phenotype in all three intestinal segments of Lal KO mice, accompanied by a higher expression of GPNMB and concentrations of circulating sTREM2. However, only duodenal macrophages activated a metabolic switch from lipids to other pathways, which were downregulated in the jejunum and ileum of Lal KO mice. Our results provide new insights into the process of absorption in control mice and possible novel markers of LAL-D and/or systemic inflammation in LAL-D.
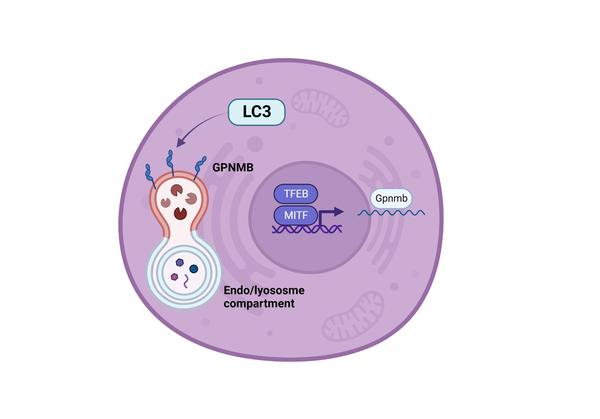
Glycoprotein Non-Metastatic Protein B (GPNMB): The Missing Link Between Lysosomes and Obesity
As a result of an unhealthy diet and limited physical activity, obesity has become a widespread pandemic worldwide and is an important predictor for the development of cardiovascular disease. Obesity is often characterized by a pro-inflammatory environment in white adipose tissue (WAT), mainly due to increased macrophage infiltration. These immune cells boost their lipid concentrations by accumulating the content of dying adipocytes. As the lysosome is highly involved in lipid handling, the progressive lipid accumulation may result in lysosomal stress and a metabolic shift. Recent studies have identified glycoprotein non-metastatic melanoma protein B (GPNMB) as a novel marker of inflammatory diseases. GPNMB is a type I transmembrane protein on the cell surface of various cell types, such as macrophages, dendritic cells, osteoblasts, and microglia, from which it can be proteolytically cleaved into a soluble molecule. It is induced by lysosomal stress via microphthalmia-associated transcription factor and thus has been found to be upregulated in many lysosomal storage disorders. In addition, a clear connection between GPNMB and obesity was recently established. GPNMB was shown to have protective and anti-inflammatory effects in most cases, preventing the progression of obesity-related metabolic disorders. In contrast, soluble GPNMB likely has the opposite effect and promotes lipogenesis in WAT. This review aims to summarize and clarify the role of GPNMB in the progression of obesity and to highlight its potential use as a biomarker for lipid-associated disorders.

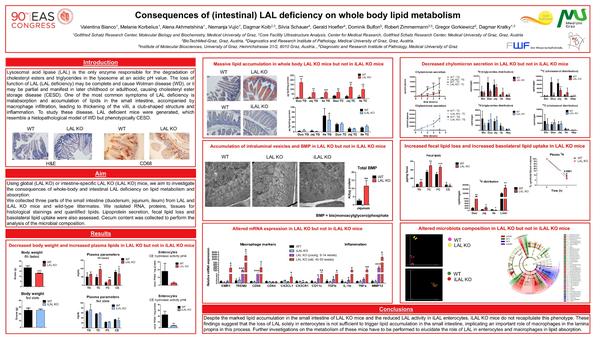
Impact of (intestinal) LAL deficiency on lipid metabolism and macrophage infiltration
- Impaired lipid absorption is a major pathological feature of LAL deficiency.
- LAL KO mice suffer from dietary lipid malabsorption.
- Basolaterally derived lipids get stuck in lamina propria macrophages in LAL KO mice.
- Enterocyte-specific LAL KO mice do not phenocopy global LAL KO mice.
- Macrophages are the key players of the intestinal phenotype in LAL deficiency.
- LAL KO mice suffer from dietary lipid malabsorption.
- Basolaterally derived lipids get stuck in lamina propria macrophages in LAL KO mice.
- Enterocyte-specific LAL KO mice do not phenocopy global LAL KO mice.
- Macrophages are the key players of the intestinal phenotype in LAL deficiency.
Poster European Atherosclerosis Society 2022
Consequences of (intestinal) LAL deficiency on whole body lipid metabolism
Poster European Lipoprotein Club 2023
Drastic differences between intestinal tracts of WT mice and mice deficient in lysosomal acid lipase

